Combination of fluorescent switch and electrochemical switch based on a photochromic diarylethene†
Abstract
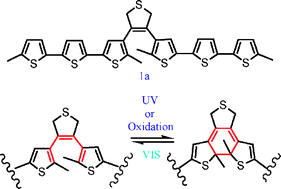
A new photochromic diarylethene with oligothiophene side arm substituents has been synthesized. This diarylethene undergoes excellent photoisomerization with UV/Vis light irradiation, and shows fluorescence emission and electrochemical behavior. Both the fluorescence emission and oxidation/

 Please wait while we load your content...
Please wait while we load your content...