Catechol O-methylation with dimethyl carbonate over different acid–base catalysts
Abstract
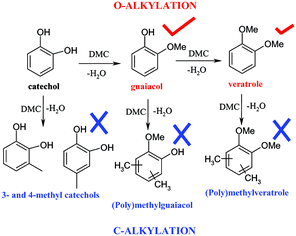
A series of meso and microporous materials, previously described and characterised, were tested in the catechol O-alkylation process using methanol (MeOH) and dimethyl carbonate (DMC) as alkylating reagents. In this regard, interesting results in terms of catalytic activity and selectivity to the desired monomethylated product (guaiacol) compared to the dimethylated one (veratrole) were found for the majority of the catalysts. Moreover, DMC is a better methylating agent than methanol with respect to the conversion ratio of catechol and guaiacol. The presence of n-type nucleophilic centres (oxygen from the OH groups) together with π-type ones (aromatic ring) in catechol led only to O-alkylated (guaiacol and veratrole), whereas no C-alkylated products were found under the reaction conditions. AlPO4 and, especially, AlPO4–Al2O3 systems showed the best performance in this alkylation process in comparison with silicoaluminophosphates (SAPO) and some acidic commercial zeolites (H-Y, H-β and H-ZSM-5).

 Please wait while we load your content...
Please wait while we load your content...