Experimental determination of the conformational free energies (A values) of fluorinated substituents in cyclohexane by dynamic 19F NMR spectroscopy. Part 1. Description of the method for the trifluoromethyl group†
Abstract
The conformational free energy (A value) of the
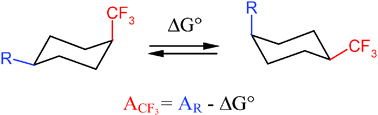

 Please wait while we load your content...
Please wait while we load your content...