Valence shell charge concentrations and the Dewar–Chatt–Duncanson bonding model†
Abstract
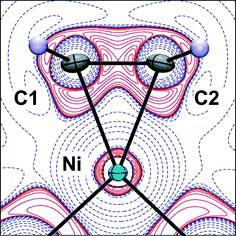
Combined experimental and theoretical charge density studies of the complex [Ni(η2-C2H4)dbpe] (dbpe = But2PCH2CH2PBut2), 1, reveal how the location and magnitude of charge concentrations in the valence shell of the metal atom influence the σ- and π-components of the metal–

 Please wait while we load your content...
Please wait while we load your content...