Synthesis and photochromism of two new 1,2-bis(thiazolyl)perfluorocyclopentenes with chelating sites
Abstract
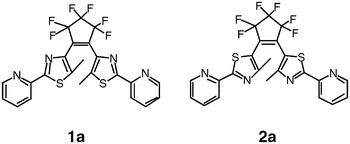
Two new dithiazolylethenes, 1a
{1,2-bis[5′-methyl-2′-(2′′-pyridyl)thiazolyl]perfluorocyclopentene} and 2a
{1,2-bis[4′-methyl-2′-(2′′-pyridyl)thiazolyl]perfluorocyclopentene}, have been synthesized. Their photochromic behavior has been fully investigated in solution as well as in the crystalline phase. They display both fatigue-resistant and thermally irreversible photochromic reactions, with more than 80% of the closed-ring forms (1b and 2b, respectively) in the photo-stationary state. Upon UV irradiation, a colorless solution of 1a turns purple while the yellow color of a 2a solution becomes more intense. Such marked differences in their

 Please wait while we load your content...
Please wait while we load your content...