Cycloaddition reactions of hydrofullerenes with cyano-substituted alkenes under basic conditions
Abstract
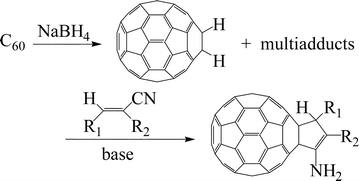
Hydrogenated [60]fullerenes, prepared from [60]fullerene and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCN(CO2Et): R = C6H5
(2a), 4-CH3O–C6H4
(2b), 4-NO2–C6H4
(2c), H (2d)] and alkylidenemalononitriles [RCH
CCN(CO2Et): R = C6H5
(2a), 4-CH3O–C6H4
(2b), 4-NO2–C6H4
(2c), H (2d)] and alkylidenemalononitriles [RCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2: R = C6H5
(2e), 4-CH3O–C6H4
(2f), 4-NO2–C6H4
(2g), 4-(CH3)2N–C6H4
(2h)] under basic conditions to afford cyclopentenylfullerenes 3. No multi-cycloaddition products are obtained. Several bases including organic and inorganic bases can be utilized in these reactions. It is proposed that the reactions take place via the Michael addition of C60H−, generated in situ by deprotonation of dihydrofullerene with a mild base, to the electrophilic carbon-carbon double bond of substrate 2, followed by intramolecular
C(CN)2: R = C6H5
(2e), 4-CH3O–C6H4
(2f), 4-NO2–C6H4
(2g), 4-(CH3)2N–C6H4
(2h)] under basic conditions to afford cyclopentenylfullerenes 3. No multi-cycloaddition products are obtained. Several bases including organic and inorganic bases can be utilized in these reactions. It is proposed that the reactions take place via the Michael addition of C60H−, generated in situ by deprotonation of dihydrofullerene with a mild base, to the electrophilic carbon-carbon double bond of substrate 2, followed by intramolecular

 Please wait while we load your content...
Please wait while we load your content...