Basicity of exceedingly strong non-ionic organic bases in acetonitrile —Verkade's superbase and some related phosphazenes
Abstract
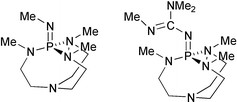
The basicity of Verkade's superbase (12) in MeCN solution is considered by a quite accurate theoretical model. It is shown that the corresponding pKa value is 29.0. Hence, its basicity is comparable or higher than that of some other P1 phosphazenes, but it is lower than the basicity of P2 phosphazenes. Structural characteristics of Verkade's superbase and its conjugate acid, as well as the origin of its pronounced basicity, are briefly discussed. Extended Verkade's superbase 13 and some Janus-type phosphazenes are examined too. It is shown that they are very good candidates for even stronger neutral organic superbases. A very useful by-product of the present study are quite accurate estimates of the gas phase proton affinities of some P1, P2, P3 and P4 polyaminophosphazenes obtained by the B3LYP/6-311+G(2df,p)//B3LYP/6-31G* scheme. The latter was successfully tested against G2 results on small molecules. This is of importance, because the experimentally measured gas phase values for phosphazenes are not available, implying that the theoretical data fill this gap with reliable information.

 Please wait while we load your content...
Please wait while we load your content...