The first one-dimensional copper(ii)-radical system with alternating double end-on and end-to-end azido bridges
Abstract
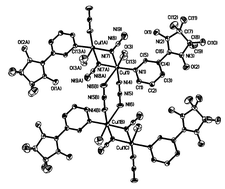
A novel one-dimensional copper(II)-radical complex [Cu(NITmPy)(N3)2(CH3OH)] [NITmPy = 2-(3′-pyridyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide] has been synthesized and structurally characterized. The structure consists of neutral chains of copper(II) ions alternatively bridged by double symmetric end-on and asymmetric end-to-end azide groups. The NITmPy radical

 Please wait while we load your content...
Please wait while we load your content...