Anisotropic thermal expansion in 18-crown-6·2 H2O·2 HNO3
Abstract
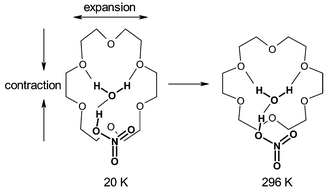
Reaction of aqueous ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) unit cell undergoes a highly anisotropic change in shape with temperature, with the crystallographic β angle changing smoothly from 66.56° to 69.03° between room temperature and 20 K. This arises from a change in shape of the
) unit cell undergoes a highly anisotropic change in shape with temperature, with the crystallographic β angle changing smoothly from 66.56° to 69.03° between room temperature and 20 K. This arises from a change in shape of the

 Please wait while we load your content...
Please wait while we load your content...