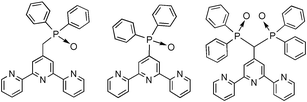
The terpyridine–phenylphosphine oxide ligands 2–4 and their Eu3+ complexes have been synthesized and studied. In acetonitrile solutions at room temperature, the complexes show an absorption band in the 300–350 nm region, indicating that the terpy units are coordinated to the metal. In these conditions, the complexes show a metal-centred luminescence, indicative of an energy transfer from the terpy subunits to the Eu centre. The efficiency of such an energy transfer process drastically depends on the nature of the anions: a 20-fold increase of the luminescence intensity could be observed upon adding up to 3 equiv. of nitrate to a solution containing equimolar amounts of ligand 2 and Eu(OTf)3. In other solvents, such as methanol, DMF, and DMSO, the metal ion is coordinated through the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group; in these conditions almost no metal-centred luminescence can be observed, showing that the efficiency of the energy transfer process from uncoordinated terpy units is almost negligible. The translocation of the Eu ion from the terpy unit to the P
O group; in these conditions almost no metal-centred luminescence can be observed, showing that the efficiency of the energy transfer process from uncoordinated terpy units is almost negligible. The translocation of the Eu ion from the terpy unit to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group can also be performed on adding Zn2+ ions; the luminescence typical of the lanthanide ion disappears, while the typical fluorescence of Zn–terpy complexes is observed.
O group can also be performed on adding Zn2+ ions; the luminescence typical of the lanthanide ion disappears, while the typical fluorescence of Zn–terpy complexes is observed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group; in these conditions almost no metal-centred luminescence can be observed, showing that the efficiency of the energy transfer process from uncoordinated terpy units is almost negligible. The translocation of the Eu ion from the terpy unit to the P
O group; in these conditions almost no metal-centred luminescence can be observed, showing that the efficiency of the energy transfer process from uncoordinated terpy units is almost negligible. The translocation of the Eu ion from the terpy unit to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group can also be performed on adding Zn2+ ions; the luminescence typical of the lanthanide ion disappears, while the typical fluorescence of Zn–terpy complexes is observed.
O group can also be performed on adding Zn2+ ions; the luminescence typical of the lanthanide ion disappears, while the typical fluorescence of Zn–terpy complexes is observed.

 Please wait while we load your content...
Please wait while we load your content...