Synthesis of a transient tropylidene substituted N-heterocyclic carbene (tropNHC): rearrangement and formation of its gold complex†
Abstract
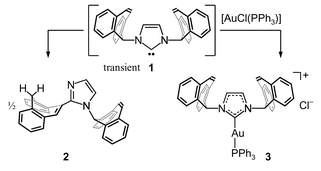
The condensation reaction of the primary tropylidenyl amine tropamine 2 [IUPAC: (5H-dibenzo[a,d]cyclohepten-5-yl)amine] with glyoxal, OHC–CHO (3), leads to the corresponding 1,4-diazadiene bistropdad 4 [IUPAC: 1,4-bis(5H-dibenzo[a,d]cyclohepten-5-yl)-1,4-diazabuta-1,3-diene] in high yield. With formaldehyde and ethereal HCl, 4 is transformed to the bistropimidazolium salt 5 [IUPAC: 1,3-bis(5H-dibenzo[a,d]cycloheptenyl)imidazolium chloride]. Deprotonation with KOtBu in thf did not gave a stable N-heterocyclic carbene bistropNHC 6 but the imidazole derivative 2-(5H-dibenzo[a,d]cyclohepten-10-yl)-1-(5H-dibenzo[a,d]cyclohepten-5-yl)-1H-imidazole 9 as a product of a rearrangement. However, the unstable carbene 6 can be trapped when it is generated in the presence of [AuCl(PPh3)] whereby the stable cationic mixed phosphane carbene gold complex {[1,3-bis(5H-dibenzo[a,d]cycloheptenyl)imidazol-2-ylidene][triphenylphosphine]gold(I)} chloride 10 is obtained which was characterised by X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...