Nonsolvent induced reconfigurable bonding configurations of ligands in nanoparticle purification†
Abstract
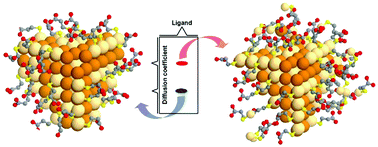
The geometry of the organic ligands on colloidal nanoparticles (NPs) is central for understanding the self-assembly behavior and many properties of NP-based soft matter. However, the current models of the NP–media interface give incomplete and often incorrect representations of its structure, especially for aqueous systems. Here, we show that NPs capped with 3-mercaptopropionic acid (MPA) serve as a convenient model for elucidation of surface ligand geometry and its effect on collective noncovalent forces of highly concentrated NPs. Contrary to expectations, MPA was found to form surface layers with different thicknesses varying from monolayer to multilayers depending on the ratio of isopropanol to the as-made NP dispersions in the purification. Solution-state nuclear magnetic resonance (NMR) and theoretical simulations uncovered that the monolayer includes strongly coordinated ligands and adsorbed ligands interacting with the NP surface non-covalently. Increased density of the non-covalently attached ligands results in a multilayered ligand shell. Consequentially, we demonstrate that the viscoelasticity of concentrated NP dispersions carrying a monolayer of MPA is higher than those of multilayered NPs. These observations enable a better understanding of the complexity of water-soluble NP dispersions and collective non-covalent forces of supramolecular NPs.


 Please wait while we load your content...
Please wait while we load your content...