Extraordinary thermal behavior of graphene oxide in air for electrode applications†
Abstract
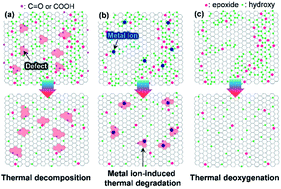
The thermal stability of solution-exfoliated graphene oxide (GO) in air is one of the most important physical properties influencing its potential applications. To date, the majority of the GO prepared by the KMnO4-based oxidation of graphite is thermally unstable in air due to the presence of highly oxidative functional groups, such as carboxyl and lactol groups that possess defective basal plane structures. Here, we demonstrate that less defective and metal ion-free GO nanosheets including those with a high oxidation level can remain very stable even above 300 °C under ambient conditions. These GO nanosheets were produced by the exfoliation of graphite oxide fabricated by the modified Brodie method in NH4OH solution, effectively excluding metal ions that can promote the thermal decomposition of GO in air at elevated temperatures. The deoxygenation of ammonia-assisted GO (AGO) was initiated at temperatures above 200 °C, while GO exfoliated in the KOH solution (KGO) decomposed, even at 180 °C. Notably, AGO was exceptionally resistant at 400 °C, even at a very slow heating rate of 2 °C min−1. Conversely, KGO was significantly oxidized, even at 250 °C. The superior thermal stability of AGO is favorable for the fabrication of conductive surface graphene films and conductive fibers by low-temperature annealing.


 Please wait while we load your content...
Please wait while we load your content...