Phase transformation in tungsten oxide nanoplates as a function of post-annealing temperature and its electrochemical influence on energy storage†
Abstract
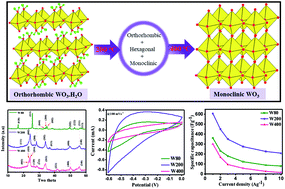
The morphology and crystal structure of electrode materials have an enormous impact on their electrochemical properties for employment in supercapacitors for various applications. In this study, the transformations of the crystal structure of WO3·H2O nanoplates were conducted by post-annealing at 200 °C and 400 °C. The morphological and structural evolution of the electrodes was studied via FEG-SEM, HRTEM, FTIR, XRD, and Raman spectroscopy. The phase transition and enhanced degree of crystallinity were observed with increasing temperature. The orthorhombic structures of the hydrate WO3·H2O (W80), the mixed-phase with mesoporous structure (W200), and finally the monoclinic phase of WO3 structures (W400) were achieved at annealing temperatures of 80 °C, 200 °C, and 400 °C respectively. The electrochemical performance of electrode W200 showed the highest specific capacitance of 606 F g−1 as compared to electrode W80 (361 F g−1), and was two-fold greater than electrode W400 (302 F g−1) at a current density of 1 A g−1. Moreover, electrode W200 exhibited excellent cyclic stability of 89% at an ultrahigh scan rate of 100 mV s−1 after 4000 cycles. The results highlight that the mixed-phase WO3 nanoplates would make a suitable electrode material for supercapacitors with desired electrochemical features.


 Please wait while we load your content...
Please wait while we load your content...