Nanobodies against the metal binding domains of ATP7B as tools to study copper transport in the cell†
Abstract
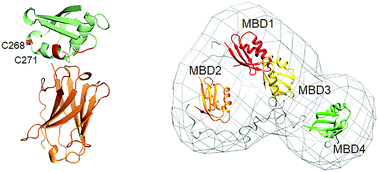
Nanobodies are genetically engineered single domain antibodies derived from the unusual heavy-chain only antibodies found in llamas and camels. The small size of the nanobodies and flexible selection schemes make them uniquely versatile tools for protein biochemistry and cell biology. We have developed a panel of nanobodies against the metal binding domains of the human copper transporter ATP7B, a multidomain membrane protein with a complex regulation of enzymatic activity and intracellular localization. To enable the use of the nanobodies as tools to investigate copper transport in the cell, we characterized their binding sites and affinity by isothermal titration calorimetry and NMR. We have identified nanobodies against each of the first four metal binding domains of ATP7B, with a wide affinity range, as evidenced by dissociation constants from below 10−9 to 10−6 M. We found both the inhibitory and activating nanobodies among those tested. The diverse properties of the nanobodies make the panel useful for the structural studies of ATP7B, immunoaffinity purification of the protein, modulation of its activity in the cell, protein dynamics studies, and as mimics of copper chaperone ATOX1, the natural interaction partner of ATP7B.


 Please wait while we load your content...
Please wait while we load your content...
