Fate of cisplatin and its main hydrolysed forms in the presence of thiolates: a comprehensive computational and experimental study†
Abstract
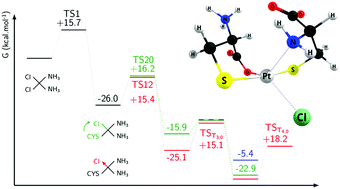
Interaction of platinum-based drugs with proteins containing sulphur amino acids is usually argued as one of the major reasons for the observed resistance to these drugs, mainly due to the deactivation of the native compounds by very efficient thiolation processes in the organism. In this work, we have investigated the detailed thermodynamics and kinetics of reaction between cisplatin cis-[PtCl2(NH3)2] and its major hydrolysed forms (monohydroxocisplatin cis-[PtCl(OH)(NH3)2] and monoaquacisplatin cis-[PtCl(H2O)(NH3)2]+) with various thiolates (methanethiolate, cysteine and glutathione) and methionine. We have used a demanding quantum chemistry approach at the MP2 and DFT levels of theory to determine the Gibbs free energies and the barrier of reactions of the most possible reaction paths. The substitution of the four ligands of the complexes studied here (Cl−, OH−, H2O and NH3) can either proceed by direct thiolations or bidentations. Our Raman spectroscopy measurements show that only two thiolations actually occur, although four are possible in principle. The reason could lie in the bidentation reactions eventually taking place after each thiolation, which is backed up by our computational results. The observed lability scale of the ligands under thiolate exposure was found to be in the following order H2O > Cl− ≈ NH3(trans) > NH3(cis) > OH−, the difference between ammine ligands being induced by a significant trans-labilization by thiolates. Finally, the S,N bidentation is shown to be preferred with respect to the S,O one.


 Please wait while we load your content...
Please wait while we load your content...