Iron transitions during activation of allosteric heme proteins in cell signaling
Abstract
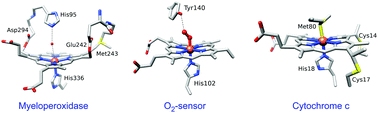
Allosteric heme proteins can fulfill a very large number of different functions thanks to the remarkable chemical versatility of heme through the entire living kingdom. Their efficacy resides in the ability of heme to transmit both iron coordination changes and iron redox state changes to the protein structure. Besides the properties of iron, proteins may impose a particular heme geometry leading to distortion, which allows selection or modulation of the electronic properties of heme. This review focusses on the mechanisms of allosteric protein activation triggered by heme coordination changes following diatomic binding to proteins as diverse as the human NO-receptor, cytochromes, NO-transporters and sensors, and a heme-activated potassium channel. It describes at the molecular level the chemical capabilities of heme to achieve very different tasks and emphasizes how the properties of heme are determined by the protein structure. Particularly, this reviews aims at giving an overview of the exquisite adaptability of heme, from bacteria to mammals.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...