Organoselenium compounds as mimics of selenoproteins and thiol modifier agents†
Abstract
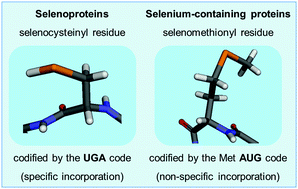
Selenium is an essential trace element for animals and its role in the chemistry of life relies on a unique functional group: the selenol (–SeH) group. The selenol group participates in critical redox reactions. The antioxidant enzymes glutathione peroxidase (GPx) and thioredoxin reductase (TrxR) exemplify important selenoproteins. The selenol group shares several chemical properties with the thiol group (–SH), but it is much more reactive than the sulfur analogue. The substitution of S by Se has been exploited in organic synthesis for a long time, but in the last 4 decades the re-discovery of ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one) and the demonstration that it has antioxidant and therapeutic properties has renovated interest in the field. The ability of ebselen to mimic the reaction catalyzed by GPx has been viewed as the most important molecular mechanism of action of this class of compound. The term GPx-like or thiol peroxidase-like reaction was previously coined in the field and it is now accepted as the most important chemical attribute of organoselenium compounds. Here, we will critically review the literature on the capacity of organoselenium compounds to mimic selenoproteins (particularly GPx) and discuss some of the bottlenecks in the field. Although the GPx-like activity of organoselenium compounds contributes to their pharmacological effects, the superestimation of the GPx-like activity has to be questioned. The ability of these compounds to oxidize the thiol groups of proteins (the thiol modifier effects of organoselenium compounds) and to spare selenoproteins from inactivation by soft-electrophiles (MeHg+, Hg2+, Cd2+, etc.) might be more relevant for the explanation of their pharmacological effects than their GPx-like activity. In our view, the exploitation of the thiol modifier properties of organoselenium compounds can be harnessed more rationally than the use of low mass molecular structures to mimic the activity of high mass macromolecules that have been shaped by millions to billions of years of evolution.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...