Alpha-synuclein: relating metals to structure, function and inhibition
Abstract
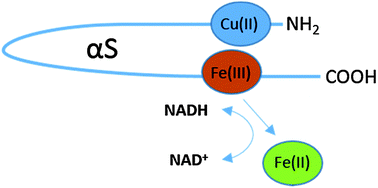
Alpha-synuclein has long been studied due to its involvement in the progression of Parkinson's disease (PD), a common neurodegenerative disorder, although a consensus on the exact function of this protein is elusive. This protein shows remarkable structural plasticity and this property is important for both correct cellular function and pathological progression of PD. Formation of intracellular oligomeric species within the substantia nigra correlates with disease progression and it has been proposed that formation of a partially folded intermediate is key to the initiation of the fibrillisation process. Many factors can influence changes in the structure of alpha-synuclein such as disease mutations and interaction with metals and neurotransmitters. High concentrations of both dopamine and metals are present in the substantia nigra making this an ideal location for both the structural alteration of alpha-synuclein and the production of toxic oxygen species. The recent proposal that alpha-synuclein is a ferrireductase is important as it can possibly catalyse the formation of such reactive species and as a result exacerbate neurodegeneration.

 Please wait while we load your content...
Please wait while we load your content...