Inhibition of human DNA topoisomerase IB by nonmutagenic ruthenium(ii)-based compounds with antitumoral activity†
Abstract
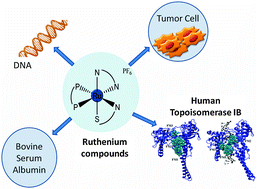
Herein we synthesized two new ruthenium(II) compounds [Ru(pySH)(bipy)(dppb)]PF6 (1) and [Ru(HSpym)(bipy)(dppb)]PF6 (2) that are analogs to an antitumor agent recently described, [Ru(SpymMe2)(bipy)(dppb)]PF6 (3), where [(Spy) = 2-mercaptopyridine anion; (Spym) = 2-mercaptopyrimidine anion and (SpymMe2) = 4,6-dimethyl-2-mercaptopyrimidine anion]. In vitro cell culture experiments revealed significant anti-proliferative activity for 1–3 against HepG2 and MDA-MB-231 tumor cells, higher than the standard anti-cancer drugs doxorubicin and cisplatin. No mutagenicity is detected when compounds are evaluated by cytokinesis-blocked micronucleus cytome and Ames test in the presence and absence of S9 metabolic activation from rat liver. Interaction studies show that compounds 1–3 can bind to DNA through electrostatic interactions and to albumin through hydrophobic interactions. The three compounds are able to inhibit the DNA supercoiled relaxation mediated by human topoisomerase IB (Top1). Compound 3 is the most efficient Top1 inhibitor and the inhibitory effect is enhanced upon pre-incubation with the enzyme. Analysis of different steps of Top1 catalytic cycle indicates that 3 inhibits the cleavage reaction impeding the binding of the enzyme to DNA and slows down the religation reaction. Molecular docking shows that 3 preferentially binds closer to the residues of the active site when Top1 is free and lies on the DNA groove downstream of the cleavage site in the Top1–DNA complex. Thus, 3 can be considered in further studies for a possible use as an anticancer agent.

 Please wait while we load your content...
Please wait while we load your content...