Variable primary coordination environments of Cd(ii) binding to three helix bundles provide a pathway for rapid metal exchange†
Abstract
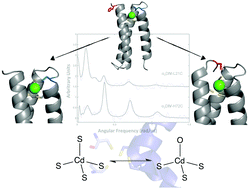
Members of the ArsR/SmtB family of transcriptional repressors, such as CadC, regulate the intracellular levels of heavy metals like Cd(II), Hg(II), and Pb(II). These metal sensing proteins bind their target metals with high specificity and affinity, however, a lack of structural information about these proteins makes defining the coordination sphere of the target metal difficult. Lingering questions as to the identity of Cd(II) coordination in CadC are addressed via protein design techniques. Two designed peptides with tetrathiolate metal binding sites were prepared and characterized, revealing fast exchange between CdS3O and CdS4 coordination spheres. Correlation of 111mCd PAC spectroscopy and 113Cd NMR spectroscopy suggests that Cd(II) coordinated to CadC is in fast exchange between CdS3O and CdS4 forms, which may provide a mechanism for rapid sensing of heavy metal contaminants by this regulatory protein.

 Please wait while we load your content...
Please wait while we load your content...