Kinetic results for mutations of conserved residues H304 and R309 of human sulfite oxidase point to mechanistic complexities†
Abstract
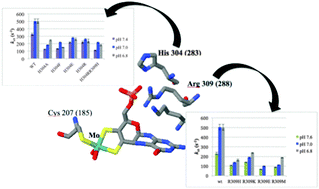
Several point mutations in the gene of human sulfite oxidase (hSO) result in isolated sulfite oxidase deficiency, an inherited metabolic disorder. Three conserved residues (H304, R309, K322) are hydrogen bonded to the phosphate group of the molybdenum cofactor, and the R309H and K322R mutations are responsible for isolated sulfite oxidase deficiency. The kinetic effects of the K322R mutation have been previously reported (Rajapakshe et al., Chem. Biodiversity, 2012, 9, 1621–1634); here we investigate several mutants of H304 and R309 by steady-state kinetics, laser flash photolysis studies of intramolecular electron transfer (IET), and spectroelectrochemistry. An unexpected result is that all of the mutants show decreased rates of IET but increased steady-state rates of catalysis. However, in all cases the rate of IET is greater than the overall turnover rate, showing that IET is not the rate determining step for any of the mutations.

 Please wait while we load your content...
Please wait while we load your content...