Imposing function down a (cupin)-barrel: secondary structure and metal stereochemistry in the αKG-dependent oxygenases
Abstract
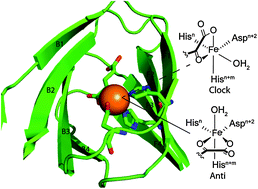
The Fe(II)/αketoglutarate (αKG) dependent oxygenases catalyze a diverse range of reactions significant in biological processes such as antibiotic biosynthesis, lipid metabolism, oxygen sensing, and DNA and RNA repair. Although functionally diverse, the eight-stranded β-barrel (cupin) and HX(D/E)XnH facial triad motifs are conserved in this super-family of enzymes. Crystal structure analysis of 25 αKG oxygenases reveals two stereoisomers of the Fe cofactor, Anti and Clock, which differ in the relative position of the exchangeable ligand position and the primary substrate. Herein, we discuss the relationship between the chemical mechanism and the secondary coordination sphere of the αKG oxygenases, within the constraints of the stereochemistry of the Fe cofactor. Sequence analysis of the cupin barrel indicates that a small subset of positions constitute the second coordination sphere, which has significant ramifications for the structure of the ferryl intermediate. The competence of both Anti and Clock stereoisomers of Fe points to a ferryl intermediate that is 5 coordinate. The small number of conserved close contacts within the active sites of αKG oxygenases can be extended to chemically related enzymes, such as the αKG-dependent halogenases SyrB2 and CytC3, and the non-αKG dependent dioxygenases isopenicillin N synthase (IPNS) and cysteine dioxygenase (CDO).
- This article is part of the themed collection: Microbial Metallomics

 Please wait while we load your content...
Please wait while we load your content...