The effect of Cu2+ and Zn2+ on the Aβ42peptide aggregation and cellular toxicity
Abstract
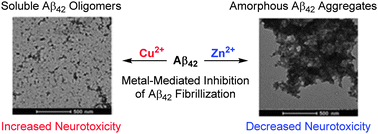
The coordination chemistry of Cu and Zn metal ions with the amyloid β (Aβ)
Maintenance work is planned for Wednesday 1st May 2024 from 9:00am to 11:00am (BST).
During this time, the performance of our website may be affected - searches may run slowly and some pages may be temporarily unavailable. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.
* Corresponding authors
a
Department of Chemistry, Washington University, One Brookings Drive, St. Louis, Missouri 63130-4899, USA
E-mail:
mirica@wustl.edu
b Department of Neurology, Washington University School of Medicine, St. Louis, Missouri 63108, USA
The coordination chemistry of Cu and Zn metal ions with the amyloid β (Aβ)

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
