X-ray reduction correlates with soaking accessibility as judged from four non-crystallographically related diiron sites†
Abstract
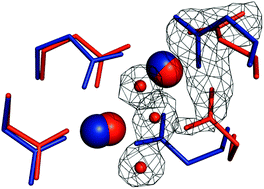
X-ray crystallography is extensively used to determine the atomic structure of proteins and their cofactors. Though a commonly overlooked problem, it has been shown that structural damage to a redox active metal site may precede loss of diffractivity by more than an order of magnitude in X-ray dose. Therefore the risk of misassigning redox states is great. Adequate treatment and consideration of this issue is of paramount importance in metalloprotein science, from experimental design to interpretation of the data and results. Some metal sites appear to be much more amenable to reduction than others, but the underlying processes are poorly understood. Here, we have analyzed the four non-crystallographically related diiron sites in a crystal of the ribonucleotide reductase R2F protein from Corynebacterium ammoniagenes. We conclude that the amount of X-ray reduction a metal site suffers correlates with its soaking accessibility. This direct observation supports the hypothesis that a diffusion component is involved in the X-ray reduction process.
- This article is part of the themed collection: Metallomics Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...