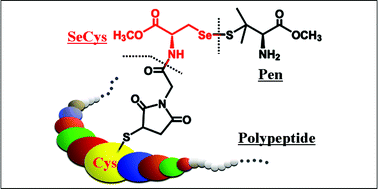
Because the seleno-L-cysteine (SeCys or Sec) insertion into selenoproteins occurs by a specific translational control process, it is quite difficult to express the SeCys-containing polypeptides even by the state-of-the-art genetic engineering techniques. In this paper, we describe a convenient synthetic method for the selective introduction of a SeCys derivative to polypeptides under physiological conditions. One SeCys residue in the seleno-L-cystine (SeCys-Se-Se-SeCys) methyl ester was first substituted with the Boc-protected penicillamine (Pen) methyl ester to form selenenylsulfide (SeCys-Se-S-Pen), an intermediate in the cellular glutathione peroxidase (GPx) catalytic cycle. Subsequently, the SeCys-Pen was coupled with the thiol-specific N-carboxymethylmaleimide through the α-amino group of the SeCys {[2-(N-maleimidyl)-1-oxo-ethyl-SeCys-methyl-Se-yl]-S-Pen methyl ester, MOE-SeCys-Pen}. The use of the MOE-SeCys-Pen allowed the selective introduction of the SeCys moiety to human serum albumin by alkylation of the thiol at its cysteine34, which generated the GPx-like activity responsible for the selenium atom in the MOE-SeCys-Pen. Consequently, this synthetic method will allow generating SeCys-containing artificial polypeptides with a GPx-like activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...