Reprogramming of cancer invasiveness and macrophage education via a nanostructured antagonist of the TGFβ receptor†
Abstract
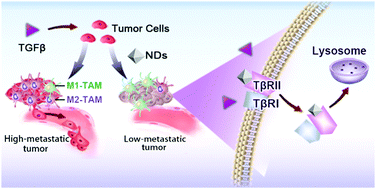
Nanoparticles (NPs) can interact with a large variety of endogenous proteins upon entering a living system. However, the effects of NP adsorption on protein functions and consequent cellular behaviors are poorly understood. Here, a mass spectrometry analysis is applied to delineate a proteome-scale map of the nanodiamond (ND) interactome network in cancer cells, which identifies the transforming growth factor β (TGFβ) type II receptor (TβRII) as a high affinity binding partner. Further investigation shows that NDs promote the lysosomal degradation of TβRII, and thereby suppress the invasion and metastasis of cultured cancer cells, tumor organoids and xenograft tumors via the blockade of the TGFβ signaling cascade. Significantly, intravenous administration of NDs reduces the recruitment of tumor-associated macrophages (TAMs) and inhibits M2 macrophage polarization in the tumor microenvironment. This study thus reveals NDs as a type of receptor antagonist and suggests their therapeutic effect in cancer treatment.


 Please wait while we load your content...
Please wait while we load your content...