The impact of cation–π, anion–π, and CH–π interactions on the excited-state intramolecular proton transfer of 1,4-dihydroxyanthraquinone†
Abstract
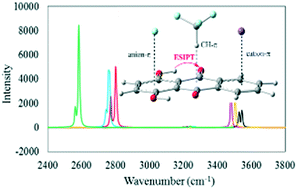
The impact of cation–π, anion–π, and CH–π interactions on the photophysical properties of quinizarin have been investigated using the density functional theory (DFT) and time-dependent density functional theory (TD-DFT) at the M06-2X/6-311++G(d,p) level in the gas phase and solution. The complexation of quinizarin with some mono- and divalent ions (including Na+, K+, Mg2+, Ca2+, F−, Cl−, and Br−) and the CF3H molecule leads to unfavorable proton transfers in the ground state. Excitation reinforces the intramolecular hydrogen bond in the complexes and facilitates proton transfer in the first excited state. The complexes exhibit exothermic excited-state proton transfer in the solution. The cation–π, anion–π, and CH–π interactions affect the absorption and emission bands and provide a wide tunable range of fluorescence emission. The large Stokes shift is an important property of the complexes that indicates their desirable fluorescence properties. The complexes involved in anion–π interactions possess the largest Stokes shift in the solution.


 Please wait while we load your content...
Please wait while we load your content...