Decoupling segmental relaxation and ionic conductivity for lithium-ion polymer electrolytes
Abstract
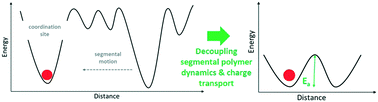
The use of polymer electrolytes instead of liquid organic systems is considered key for enhancing the safety of lithium batteries and may, in addition, enable the transition to high-energy lithium metal anodes. An intrinsic limitation, however, is their rather low ionic conductivity at ambient temperature. Nonetheless, it has been suggested that this might be overcome by decoupling the ion transport and the segmental relaxation of the coordinating polymer. Here, we provide an overview of the different approaches to achieve such decoupling, including a brief recapitulation of the segmental-relaxation dependent ion conduction mechanism, exemplarily focusing on the archetype of polymer electrolytes – polyethylene oxide (PEO). In fact, while the understanding of the underlying mechanisms has greatly improved within recent years, it remains rather challenging to outperform PEO-based electrolyte systems. Nonetheless, it is not impossible, as highlighted by several examples mentioned herein, especially in consideration of the extremely rich polymer chemistry and with respect to the substantial progress already achieved in designing tailored molecules with well-defined nanostructures.
- This article is part of the themed collection: Charge Transporting Nanostructured Polymers for Electrochemical Systems


 Please wait while we load your content...
Please wait while we load your content...