Natural product inspired optimization of a selective TRPV6 calcium channel inhibitor†
Abstract
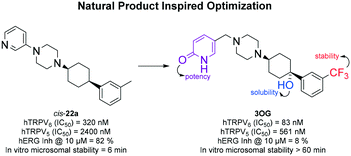
Transient receptor potential vanilloid 6 (TRPV6) is a calcium channel implicated in multifactorial diseases and overexpressed in numerous cancers. We recently reported the phenyl-cyclohexyl-piperazine cis-22a as the first submicromolar TRPV6 inhibitor. This inhibitor showed a seven-fold selectivity against the closely related calcium channel TRPV5 and no activity on store-operated calcium channels (SOC), but very significant off-target effects and low microsomal stability. Here, we surveyed analogues incorporating structural features of the natural product capsaicin and identified 3OG, a new oxygenated analog with similar potency against TRPV6 (IC50 = 0.082 ± 0.004 μM) and ion channel selectivity, but with high microsomal stability and very low off-target effects. This natural product-inspired inhibitor does not exhibit any non-specific toxicity effects on various cell lines and is proposed as a new tool compound to test pharmacological inhibition of TRPV6 mediated calcium flux in disease models.


 Please wait while we load your content...
Please wait while we load your content...