Development of potent CPP6–gemcitabine conjugates against human prostate cancer cell line (PC-3)†
Abstract
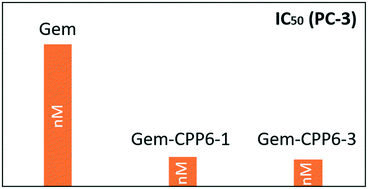
Gemcitabine (dFdC) is a nucleoside analogue used in the treatment of various cancers, being a standard treatment for advanced pancreatic cancer. The effect of gemcitabine is severely compromised due to its rapid plasma degradation, systemic toxicity and drug resistance, which restricts its therapeutic efficacy. Our main goal was to develop new active conjugates of dFdC with novel cell-penetrating hexapeptides (CPP6) to facilitate intracellular delivery of this drug. All new peptides were prepared by solid phase peptide synthesis (SPPS), purified and characterized by HPLC and LC-MS. Cell-penetrating peptides (CPP) contain a considerably high ratio of positively charged amino acids, imparting them with cationic character. Tumor cells are characterized by an increased anionic nature of their membrane surface, a property that could be used by CPP to target these cells. The BxPC-3, MCF-7 and PC-3 cancer cell lines were used to evaluate the in vitro cytotoxicity of conjugates and the results showed that conjugating dFdC with CPP6 significantly enhanced cell growth inhibitory activity on PC-3 cells, with IC50 between 14 and 15 nM. These new conjugates have potential to become new therapeutic tools for cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...