Aurantiamide-related dipeptide derivatives are formyl peptide receptor 1 antagonists†
Abstract
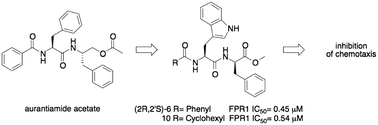
Formyl peptide receptor 1 (FPR1) is expressed on a variety of immune system cells and is a key regulator of the inflammatory environment. Therefore, the development of FPR1 antagonists may represent a novel approach for modulating innate immunity and treating inflammatory diseases. Starting from a dipeptide scaffold that is structurally related to the natural product aurantiamide, we investigated the structure–activity relationships of the dipeptide (2R,2′S)-6, which was reported as an FPR1 antagonist. We found that the absolute configuration 2R,2′S was preferred to obtain potent and selective FPR1 antagonists. The structural modifications performed on the terminal fragments of the molecule suggest that the size of the substituents can greatly influence the interaction with FPR1. These compounds behaved as antagonists in human neutrophils and were able to inhibit formyl peptide-induced chemotaxis. Since FPR1 is a key regulator of the inflammatory environment, the dipeptide derivatives described here may represent important leads for the development of new potent and selective FPR1 antagonists for the treatment of neutrophil-mediated inflammatory diseases.


 Please wait while we load your content...
Please wait while we load your content...
