Evaluation of anti-inflammatory activity and molecular docking study of new aza-bicyclic isoxazoline acylhydrazone derivatives†
Abstract
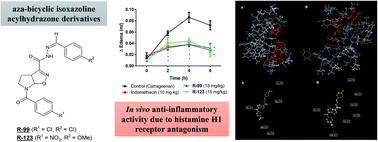
The aim of this study was to investigate the anti-inflammatory effects of two new isoxazoline-acylhydrazone derivatives: N′-(4-methoxybenzylidene)-6-(4-nitro-benzoyl)-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,2-d]isoxazole-3-carbohydrazide (R-123) and N′-(4-chlorobenzylidene)-6-(4-chlorobenzoyl)-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,2-d]isoxazole-3-carbohydrazide (R-99). An air pouch induced by carrageenan was used for screening the best dose of R-99 and R-123. Using this mouse model, leukocyte migration and cytokine levels (TNF-α and IL-1β) were determined. Paw edema induced by several phlogistic agents and vascular permeability induced by acetic acid were employed to investigate the mechanism of action of the isoxazoline-acylhydrazone derivatives. A docking study was performed with the human histamine H1 receptor to investigate potential antihistaminic activity. Treatment with the compounds reduced leukocyte migration in the air pouch at all doses tested. TNF-α and IL-1β levels were similarly reduced by the two compounds. Vasoactive amines were inhibited in models of paw edema induced by several agents and vascular permeability induced by acetic acid. The docking study suggests that R-99 and R-123 may be inhibitors of the histamine H1 receptor. In conclusion, the results indicate that R-99 and R-123 exhibit promising anti-inflammatory activity related to their ability to inhibit TNF-α, IL-1β, and vasoactive amine production, as well as reduce leukocyte migration and inhibit mast cell degranulation.


 Please wait while we load your content...
Please wait while we load your content...