Mechanism of thienopyridone and iminothienopyridinedione inhibition of protein phosphatases†
Abstract
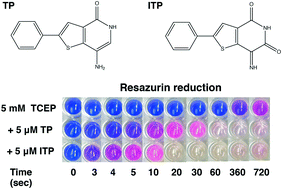
Thienopyridone (TP) has been proposed as a selective inhibitor of phosphatases of regenerating liver (PRL or PTP4A). PRLs are dual specificity phosphatases that promote cancer progression and are attractive anticancer targets. TP and iminothienopyridinedione (ITP), a more potent derivative, were shown to be effective inhibitors but the mechanism of inhibition was not established. Here, we perform NMR experiments and in vitro phosphatase assays to show that TP and ITP inhibit protein phosphatases non-specifically through oxidation of the phosphatase catalytic cysteine. We demonstrate that TP and ITP are redox active compounds, inhibiting PRL-3 and multiple other PTPs through oxidation. They also catalyze the oxidation of thioredoxin-1 as well as small molecules, like TCEP, DTT, and glutathione. The reported selectivity of TP and ITP is likely due to the higher susceptibility of PRLs to oxidation. Thus, while TP and ITP effectively inhibit PRLs, their use for studying the cellular function of PRLs is problematic due to the likelihood of off-target effects.
- This article is part of the themed collection: Molecular Pharmacology


 Please wait while we load your content...
Please wait while we load your content...