Synthesis of DNA-coupled isoquinolones and pyrrolidines by solid phase ytterbium- and silver-mediated imine chemistry†
Abstract
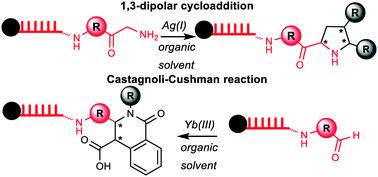
DNA-encoded libraries of chemically synthesized compounds are an important small molecule screening technology. The synthesis of encoded compounds in solution is currently restricted to a few DNA-compatible and water-tolerant reactions. Encoded compound synthesis of short DNA-barcodes covalently connected to solid supports benefits from a broad range of choices of organic solvents. Here, we show that this encoded chemistry approach allows for the synthesis of DNA-coupled isoquinolones by an Yb(III)-mediated Castagnoli–Cushman reaction under anhydrous reaction conditions and for the synthesis of highly substituted pyrrolidines by Ag(I)-mediated 1,3-dipolar azomethine ylide cycloaddition. An encoding scheme for these DNA-barcoded compounds based on a DNA hairpin is demonstrated.


 Please wait while we load your content...
Please wait while we load your content...