Vinyl sulfonamide synthesis for irreversible tethering via a novel α-selenoether protection strategy†
Abstract
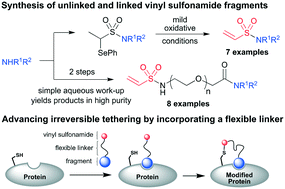
Vinyl sulfonamides are valuable electrophiles for targeted protein modification and inhibition. We describe a novel approach to the synthesis of terminal vinyl sulfonamides which uses mild oxidative conditions to induce elimination of an α-selenoether masking group. The method complements traditional synthetic approaches and typically yields vinyl sulfonamides in high purity after aqueous work-up without requiring column chromatography of the final electrophilic product. The methodology is applied to the synthesis of covalent fragments for use in irreversible protein tethering and crucially enables the attachment of diverse fragments to the vinyl sulfonamide warhead via a chemical linker. Using thymidylate synthase as a model system, ethylene glycol is identified as an effective linker for irreversible protein tethering.
- This article is part of the themed collection: Molecular Pharmacology


 Please wait while we load your content...
Please wait while we load your content...