Rationally synthesized coumarin based pyrazolines ameliorate carrageenan induced inflammation through COX-2/pro-inflammatory cytokine inhibition†
Abstract
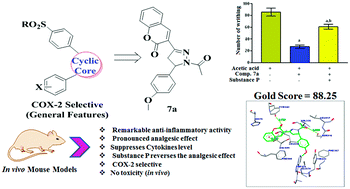
In the present work, coumarin based pyrazolines (7a–g) have been synthesized and investigated for their in vitro and in vivo anti-inflammatory potential. Amongst the synthesized compounds, compounds 7a, 7d and 7f exhibited significant in vitro anti-inflammatory activity as compared to the standard etoricoxib. Keeping this in mind, in vivo investigations were carried out via carrageenan induced inflammation and acetic acid induced writhing models in male Wistar rats and compound 7a was found to possess appreciable anti-inflammatory and analgesic potential. The mode of action of compound 7a was also investigated by using substance P as the biomarker, which shows promising results. Further, the selectivity of the most active compound 7a against the cyclooxygenase enzyme was supported by molecular docking studies which reveal that compound 7a has greater binding affinity towards COX-2 over COX-1 and 5-LOX enzymes. In silico ADME analysis of compound 7a confirms the drug-like characteristics and the in vivo acute toxicity study showed the safety of the compound even up to a 2000 mg kg−1 dose. Thus, compound 7a was identified as an effective anti-inflammatory agent, and can be explored for further analgesic/anti-inflammatory drug design and development.


 Please wait while we load your content...
Please wait while we load your content...