Antiplasmodial imidazopyridazines: structure–activity relationship studies lead to the identification of analogues with improved solubility and hERG profiles†
Abstract
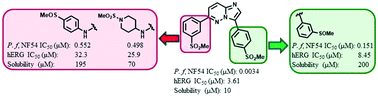
3,6-Diarylated imidazopyridazines have recently been shown to possess good in vitro antiplasmodial and in vivo antimalarial activity. However, frontrunner compounds have been associated with poor solubility and a hERG (human ether-a-go-go-related gene) inhibition liability raising concerns for potential cardiotoxicity risks. Herein, we report the synthesis and structure–activity relationship studies of new imidazopyridazines aimed at improving aqueous solubility and countering hERG inhibition while maintaining antiplasmodial potency. While we identified new analogues with potent antiplasmodial activity (IC50 = 0.031 μM against the NF54 drug-sensitive strain, and IC50 = 0.0246 μM against the K1 multidrug resistant strain), hERG inhibition remained an issue. Excitingly, on the other hand, new analogues with a substantially improved hERG inhibition profile (IC50 = 7.83–32.3 μM) with sub-micromolar antiplasmodial activity (NF54, IC50 = 0.151–0.922 μM) were identified. Similarly, the introduced molecular features also resulted in analogues with moderate to high solubility (60–200 μM) while also displaying sub-micromolar antiplasmodial potency (NF54, IC50 = 0.136–0.99 μM).


 Please wait while we load your content...
Please wait while we load your content...