Design, synthesis, and anticancer activity evaluation of irreversible allosteric inhibitors of the ubiquitin-conjugating enzyme Ube2g2†
Abstract
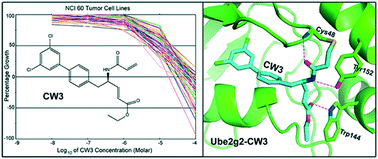
The RING finger-dependent ubiquitin ligase (E3) gp78, known as the tumor autocrine motility factor receptor, contributes to tumor progression. The protein interacts with its cognate ubiquitin-conjugating enzyme (E2), Ube2g2, via its RING domain and a unique region called G2BR that strongly binds to E2. The binding of G2BR to Ube2g2 allosterically enhances the binding of RING to E2, and the binding of RING triggers the departure of G2BR from E2 also in an allosteric fashion. Targeting these allosteric events, we developed a family of inhibitors that irreversibly block E2–E3 interactions and thereby eliminate the tumorigenic effect of gp78. One among 19 compounds screened with the NCI 60 tumor cell lines exhibited outstanding anticancer activities. At 10 μM, it caused >50% growth inhibition to 40% of the cell lines; at 100 μM, it showed lethiferous activity against most cell lines.


 Please wait while we load your content...
Please wait while we load your content...