The role of aryl-topology in balancing between selective and dual 5-HT7R/5-HT1A actions of 3,5-substituted hydantoins†
Abstract
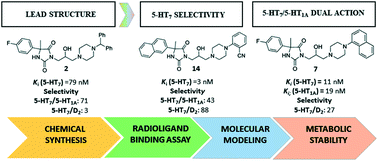
In order to search for active and selective serotonin 5-HT7R antagonists among 3,5-disubstituted arylpiperazine-imidazolidine-2,4-diones, the role of the introduction/deletion and the mutual orientation of aromatic rings was analyzed. Chemical modifications of 2nd generation lead structure of 3-(3-(4-(diphenylmethyl)piperazin-1-yl)-2-hydroxypropyl)-5-(4-fluorophenyl)-5-methylimidazolidine-2,4-dione (2, KKB16) were performed. New derivatives (4–18) were designed and synthesized. X-ray crystallographic analysis of the representative compound 5-(4-fluorophenyl)-3-[2-hydroxy-3-(4-phenylpiperazin-1-yl)propyl]-5-methylimidazolidine-2,4-dione (3) was performed to support molecular modeling and SAR studies. The affinity for 5-HT7R, D2R and 5-HT1AR in radioligand binding assays for the entire series and ADME-Tox parameters in vitro for selected compounds (7, 10, and 13) were evaluated. Molecular docking and pharmacophore model assessment were performed. According to the obtained results, 5-methyl-5-naphthylhydantoin derivatives were found to be the new highly active 5-HT7R agents (Ki ≤ 5 nM) with significant selectivity over 5-HT1AR and D2R. On the contrary, the (1-naphthyl)piperazine moiety was gained with the potent dual 5-HT7R/5-HT1AR action (Ki: 11 nM/19 nM).


 Please wait while we load your content...
Please wait while we load your content...