Chemical probes of Skp2-mediated p27 ubiquitylation and degradation
Abstract
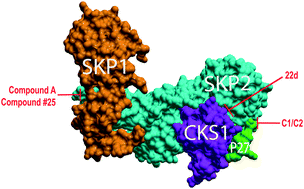
Skp2 is a member of the F-box family of proteins that serve as substrate-specific adaptors in Skp1–CUL1–ROC1–F-box (SCF) E3 ubiquitin ligases. Skp2 (Fbxl1) directly binds to the tumor suppressor p27 in the context of the SCFSkp2 E3 ubiquitin ligase to ubiquitylate and target-phosphorylated p27 for proteasomal degradation. As p27 is a powerful suppressor of growth in a variety of cells, and as Skp2 is also overexpressed in many human cancers, Skp2 is considered an oncogene and an intriguing drug target. However, despite 20 years of investigation, a valid chemical inhibitor of Skp2-mediated degradation of p27 has not been identified. Recently, an increasing number of compounds designed to have this bioactivity have been reported. Here, we conduct a meta-analysis of the evidence regarding bioactivity, structure, and medicinal chemistry in order to evaluate and compare these Skp2 inhibitor compounds. Despite chemically diverse compounds with a wide array of Skp2-mediated p27 ubiquitylation inhibition properties reported by several independent groups, no current chemical probe formally qualifies as a validated pharmaceutical hit compound. This finding suggests that our knowledge of the structural biochemistry of the Skp2–p27 complex remains incomplete and highlights the need for novel modes of inquiry.


 Please wait while we load your content...
Please wait while we load your content...