Assessment of the trifluoromethyl ketone functionality as an alternative zinc-binding group for selective HDAC6 inhibition†
Abstract
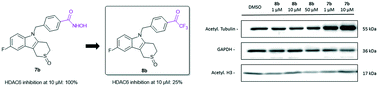
Recent studies point towards the possible disadvantages of using hydroxamic acid-based zinc-binding groups in HDAC inhibitors due to e.g. mutagenicity issues. In this work, we elaborated on our previously developed Tubathian series, a class of highly selective thiaheterocyclic HDAC6 inhibitors, by replacing the benzohydroxamic acid function by an alternative zinc chelator, i.e., an aromatic trifluoromethyl ketone. Unfortunately, these compounds showed a reduced potency to inhibit HDAC6 as compared to their hydroxamic acid counterparts. In agreement, the most active trifluoromethyl ketone was unable to influence the growth of SK-OV-3 ovarian cancer cells nor to alter the acetylation status of tubulin and histone H3. These data suggest that replacement of the zinc-binding hydroxamic acid function with a trifluoromethyl ketone zinc-binding moiety within reported benzohydroxamic HDAC6 inhibitors should not be considered as a standard strategy in HDAC inhibitor development.


 Please wait while we load your content...
Please wait while we load your content...