Synthesis and antiplasmodial activity of purine-based C-nucleoside analogues†
Abstract
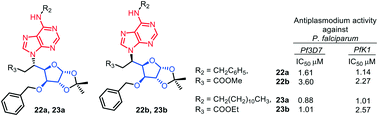
A series of homologous C-nucleoside mimics have been synthesized via an efficient and facile synthetic protocol involving the conjugate addition of purine to sugar derived olefinic ester in good yields. The synthesized compounds were evaluated for their antiplasmodial activity in vitro against both the CQ-sensitive and resistant strains of P. falciparum. Interestingly, all the synthesized nucleoside analogs exhibited an IC50 of <5 μM, while compounds 22a, 23a, and 23b showed promising antiplasmodial activity with an IC50 of 1.61, 0.88, and 1.01 μM against the CQ-sensitive Pf3D7 strain and 1.14, 1.01, and 2.57 μM against the CQ-resistant PfK1 strain, respectively.


 Please wait while we load your content...
Please wait while we load your content...