Evaluation of A-ring fused pyridine d-modified androstane derivatives for antiproliferative and aldo–keto reductase 1C3 inhibitory activity†
Abstract
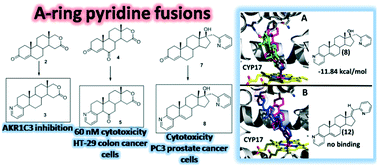
New A-ring pyridine fused androstanes in 17a-homo-17-oxa (D-homo lactone), 17α-picolyl or 17(E)-picolinylidene series were synthesized and validated by X-ray crystallography, HRMS, IR and NMR spectroscopy. Novel compounds 3, 5, 8 and 12 were prepared by treatment of 4-en-3-one or 4-ene-3,6-dione D-modified androstane derivatives with propargylamine catalyzed by Cu(II), and evaluated for potential anticancer activity in vitro using human cancer cell lines and recombinant targets of steroidal anti-cancer drugs. Pyridine fusion to position 3,4 of the A-ring may dramatically enhance affinity of 17α-picolyl compounds for CYP17 while conferring selective antiproliferative activity against PC-3 cells. Similarly, pyridine fusion to the A-ring of steroidal D-homo lactones led to identification of new inhibitors of aldo–keto reductase 1C3, an enzyme targeted in acute myeloid leukemia, breast and prostate cancers. One A-pyridine D-lactone steroid 5 also has selective submicromolar antiproliferative activity against HT-29 colon cancer cells. None of the new derivatives have affinity for estrogen or androgen receptors in a yeast screen, suggesting negligible estrogenicity and androgenicity. Combined, our results suggest that A-ring pyridine fusions have potential in modulating the anticancer activity of steroidal compounds.


 Please wait while we load your content...
Please wait while we load your content...