Conformationally restricted benzothienoazepine respiratory syncytial virus inhibitors: their synthesis, structural analysis and biological activities†
Abstract
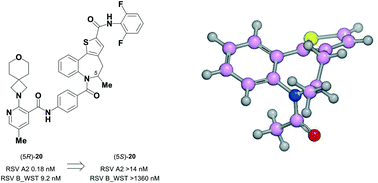
Atropisomeric drug substances are known to have different biological properties. Compounds containing the N-benzoylbenzazepine motif have been shown to exhibit energetically restricted rotation around the Ar(CO)N axis. Herein we report, for the first time, the synthesis, physical characterisation and anti-viral profiles of a series of C-4 and C-5 methylated thieno-benzazepines. NMR analysis reveals that incorporation of a single additional substituent at either of these loci influences the conformational dynamics of the azepine ring system. In the case of the C-5 alkyl analogues, the influence of the new stereocentre is so pronounced that its absolute configuration determines which unique atropisomer is obtained following the generation of the benzazepine nucleus. Screening of the alkylated derivatives for their anti-respiratory syncytial virus (RSV) activity indicates that the desired viral pathogenicity is strongly associated with the conformation adopted by the modified tricyclic scaffolds. This is particularly evident in the case of the C-5 homologues in which one atropisomer was found to be potently active and the other essentially inert. These results provide compelling evidence that we have determined the bioactive conformation shared by RSV inhibitors that employ the thienobenazapine nucleus as their core molecular architecture. Furthermore, the understanding obtained from these studies may make it possible to design improved agents against RSV infection in the future.


 Please wait while we load your content...
Please wait while we load your content...