Discovery and biological evaluation of N5-substituted 6,7-dioxo-6,7-dihydropteridine derivatives as potent Bruton's tyrosine kinase inhibitors
Abstract
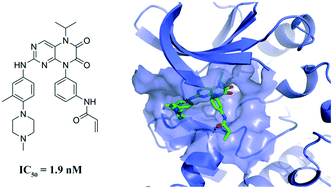
Bruton's tyrosine kinase (BTK) plays a critical role in B cell receptor (BCR)-mediated signaling pathways responsible for the development and function of B cells, which makes it an attractive target for the treatment of many types of B-cell malignancies. Herein, a series of N5-substituted 6,7-dioxo-6,7-dihydropteridine-based, irreversible BTK inhibitors were reported with IC50 values ranging from 1.9 to 236.6 nM in the enzymatic inhibition assay. Compounds 6 and 7 significantly inhibited the proliferation of Ramos cells which overexpress the BTK enzyme, as well as the autophosphorylation of BTK at Tyr223 and the activation of its downstream signaling molecule PLCγ2. Overall, this series of compounds could provide a promising starting point for further development of potent BTK inhibitors for B-cell malignancy treatment.


 Please wait while we load your content...
Please wait while we load your content...