Synthesis, characterization and biological evaluation of six highly cytotoxic ruthenium(ii) complexes with 4′-substituted-2,2′:6′,2′′-terpyridine†
Abstract
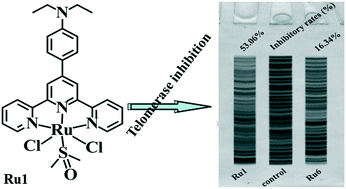
Herein, six ruthenium(II) terpyridine complexes, i.e. [RuCl2(4-EtN-Phtpy)(DMSO)] (Ru1), [RuCl2(4-MeO-Phtpy)(DMSO)] (Ru2), [RuCl2(2-MeO-Phtpy)(DMSO)] (Ru3), [RuCl2(3-MeO-Phtpy)(DMSO)] (Ru4), [RuCl2(1-Bip-Phtpy)(DMSO)] (Ru5), and [RuCl2(1-Pyr-Phtpy)(DMSO)] (Ru6) with 4′-(4-diethylaminophenyl)-2,2′:6′,2′′-terpyridine (4-EtN-Phtpy), 4′-(4-methoxyphenyl)-2,2′:6′,2′′-terpyridine (4-MeO-Phtpy), 4′-(2-methoxyphenyl)-2,2′:6′,2′′-terpyridine (2-MeO-Phtpy), 4′-(3-methoxyphenyl)-2,2′:6′,2′′-terpyridine (3-MeO-Phtpy), 4′-(1-biphenylene)-2,2′:6′,2′′-terpyridine (1-Bip-Phtpy), and 4′-(1-pyrene)-2,2′:6′,2′′-terpyridine (1-Pyr-Phtpy), respectively, were synthesized and fully characterized. The MTT assay demonstrates that the in vitro anticancer activity of Ru1 is higher than that of Ru2–Ru6 and more selective for Hep-G2 cells than for normal HL-7702 cells. In addition, various biological assays show that Ru1 and Ru6, especially the Ru1 complex, are telomerase inhibitors targeting c-myc G4 DNA and also cause apoptosis of Hep-G2 cells. With the same Ru center, the in vitro antitumor activity and cellular uptake ability of the 4-EtN-Phtpy and 1-Bip-Phtpy ligands follow the order 4-EtN-Phtpy > 1-Bip-Phtpy.


 Please wait while we load your content...
Please wait while we load your content...