Synthesis, characterization, and radioprotective activity of α,β-unsaturated aryl sulfone analogs and their Tempol conjugates†‡
Abstract
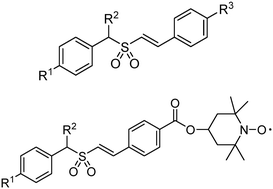
Some novel α,β-unsaturated aromatic sulfone analogs (5a–5m) and their Tempol conjugates (6a–6e) have been synthetically prepared, characterized and evaluated for their radioprotective activity under γ-ray radiation. The Tempol conjugates were characterized by X-ray single crystal diffraction. In vitro studies showed that 5a, 5b and 6b had superior activities to Ex-Rad pre-treated before 5 Gy irradiation, and 5a, 6a and 6b had better activities than Ex-Rad after 5 Gy irradiation, while 5a, 6a and 6b exhibited both prophylactic and mitigation effects, indicating the advantage of combining α,β-unsaturated aromatic sulfones with Tempol nitroxide. Both p53 and phospho-p53 levels were significantly lower in compound-treated cells than those in untreated irradiated cells. The conjugates offer an improved radioprotective ability, which is an advantage in the treatment protocol.


 Please wait while we load your content...
Please wait while we load your content...