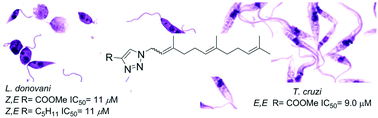
Abstract
A series of prenyl 1,2,3-triazoles were prepared from isoprenyl azides and different alkynes. The dipolar cycloaddition reaction provided exclusively primary azide products as regioisomeric mixtures that were separated by column chromatography and then fully characterized. Most of the compounds displayed antiparasitic activity against Trypanosoma cruzi and Leishmania donovani. The most active compounds were assayed as potential TcCYP51 inhibitors.


 Please wait while we load your content...
Please wait while we load your content...