Synthesis, cytotoxic and urease inhibitory activities of some novel isatin-derived bis-Schiff bases and their copper(ii) complexes†‡
Abstract
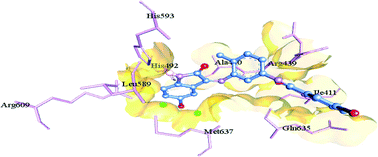
Several isatin-3-thiosemicarbazones (a class of Schiff bases) from our earlier studies have been validated as promising cytotoxic agents and urease inhibitors. Also, a number of isatin-derived imines (Schiff bases) and their Cu(II) complexes have been reported in the literature to exhibit potential cytotoxic activity towards different cells. In view of this, a series of seven new 5-(un)-substituted isatin-derived bis-Schiff bases/ligands 3a–g and their Cu(II) complexes 5a–g were synthesized and evaluated for their cytotoxic and urease inhibitory activities. All the Schiff base ligands 3a–g proved to be active in sulforhodamine B (SRB) bioassay, displaying promising cytotoxic activity against lung carcinoma (H157) cells. Compound 3b was found to be the most potent inhibitor of H157 cells, exhibiting an IC50 value of 2.32 ± 0.11 μM. Similarly, all the metal complexes 5a–g proved to be active in this assay, demonstrating enhanced cytotoxic activity in each case, occurring as a result of coordination of the Schiff base ligands to the metal ion. Compound 5d proved to be the most potent inhibitor of H157 cells, showing cytotoxic activity comparable to that of the standard drug, vincristine (VCN) (IC50 = 1.29 ± 0.06 vs. 1.03 ± 0.04 μM). In the urease inhibition assay, all the synthesized Schiff base ligands except 3f proved to be highly potent enzyme inhibitors, displaying inhibitory activity even better than that of the reference inhibitor, thiourea (IC50 = 0.04 ± 0.004–5.86 ± 0.09 vs. 22.3 ± 1.12 μM), and thus may act as promising lead molecules for further studies. Molecular docking studies were also carried out for bis-Schiff bases 3a–g to elucidate their relationship with the binding pockets of the enzyme.

 Please wait while we load your content...
Please wait while we load your content...